
برای شیرینسازی برشهای مختلف هیدروکربوری در مجتمعهای پالایشگاهی و پتروشیمی از محلول کاستیک تازه استفاده میگردد. فاضلاب حاصل شده با نام فاضلاب کاستیک مستعمل دارای بار آلایندگی زیاد از جمله، ترکیبات گوگردی، اسیدهای آلی و مقدار COD بالا بوده و جزء فاضلابهای بسیار خطرناک دستهبندی میگردد.

اسپنت کاستیک پالایشگاهی یک آلاینده ی غیر قابل تجزیه بیولوژیکی است. این فاضلاب به دلیل داشتن ویژگی های فیزیکی و شیمیایی منحصر به فرد می بایست به صورت جداگانه از سایر فاضلاب های صنعتی تصفیه شود. به دلیل وجود ملکول های بزرگ و غیر قابل تصفیه، اسپنت کاستیک توسط فرآیندهای متعارف تصفیه فاضلاب های شهری و صنعتی مانند انواع لجن فعال، قابل تصفیه نیست. در استفاده از این روشها مسائل زیست محیطی، هزینه اقتصادی و بازدهی حائز اهمیت میباشد. کاستیک مستعمل یکی از دورریزهای مایع صنایع نفت، گاز و پتروشیمی است که به دلیل محتوای سرشار از آلاینده ها، قلیائیت (۱۲> pH) ، شوری زیاد (سدیم ۵ تا ۱۲ درصد وزنی) و غلظت زیاد سولفید (۲ تا ۳ درصد وزنی) به راحتی قابل دفع به محیط زیست نیست. ترکیبات کاستیک مستعمل بسیار متنوع است و به نوع کاستیک مستعمل بستگی دارد. کاستیک مستعمل را می توان متناسب با منشا و ترکیب آنها در گروه سولفیدی، نفتنیکی و کریسیلیکی تقسیم بندی کرد.

از روش های مختلفی برای تصفیه کاستیک مستعمل بهره می گیرند که از این قرارند:
۱. خنثی سازی با سولفوریک اسید؛
۲. تزریق در چاهای عمیق؛ deep well injection
۳. انعقاد، انعقاد الکتروشیمیایی و لخته سازی؛
۴. اکسایش هوازی؛ wet air oxidation
| Classification | Temperature (Pressure) | Treatment of Compounds |
| Low | 110-150 °C
(2-10 bar) |
Reactive Sulfides |
| Mid | 200-220 °C
(20-45 bar) |
Sulfides, Mercaptans |
| High | 240-260 °C
(45-100 bar) |
Naphthenic and Cresylic Acids, Sulfides, Mercaptans |
Typical classification of WAO treatment systems
۵. اکسایش کاتالیستی؛
۶. اکسایش پیشرفته؛
۷. روش های زیستی؛
۸. روش های فیزیکی مانند تبخیر و سوزاندن نمک به جا مانده
مقایسه بعضی از روشهای تصفیه کاستیک مستعمل مطابق با تحقیقات شرکت زیمنس مندرج در کاتالوگ فصلنامه آن شرکت
| Maintenance cost | Operating cost | Capital cost | Produces Byproducts Requiring Disposal | Produces Biodegradable Effluent | Destroys Acid Oils | Reduces COD | Eliminates Odor | Technology |
| Medium | Medium | Medium | No | No | No | Yes | No | Acid springing |
| Low | Very High | Low | Yes/No | Yes | Yes | Yes | Yes | Chemical
advanced oxidation |
| Low | Low | Medium/High | No | Dependent Upon Load & WWTP | No | Yes | Mostly | Low temp
WAO |
| Low | Low | Medium/High | No | Yes | Yes | Yes | Yes | Medium temp WAO |
| Low | Low | High | No | Yes | Yes | Yes | Yes | High temp WAO |

با توجه به وجود بارهای آلی، معدنی وترکیبات معلق در ترکیب کاستیک مستعمل کل مواد جامد محلول۲ اکسیژن خواهی شیمیایی۳ این گونه ترکیبات بالاست و محدوده اکسیژن خواهی شیمیایی فاضلاب های کاستیک مستعمل در حد ۲۰۰۰ تا ۶۰۰۰۰ پی پی ام۴ است و TDS کل مواد جامد محلول این گونه ترکیبات در حد ۲۰۰۰۰۰ تا ۱۰۰۰۰۰۰ پی پی ام است و رساندن اکسیژن خواهی شیمیایی / TDS به مقادیر مورد قبول زیست محیطی به تلفیق روش های تصفیه کاستیک مستعمل نیاز دارد. با توجه به تنوع ترکیبات در این گونه فاضلاب تحقیقات نشان داد که تصفیه آنها براحتی امکان پذیر نیست . شناسایی ترکیبات کاستیک مستعمل به انتخاب فرایند تصفیه کاستیک مستعمل کمک خواهد کرد.

نتایج مقایسه نشان میدهند که، روش اکسیداسیون کاتالیستی با 34 درصد دارای بیشترین ارجحیت در میان گزینهها و معیار اقتصادی دارای 28/3 درصد ارجحیت نسبت به سایر معیارها است. نتایج شبیهسازی با توجه به دو تابع هدف تولید وزنی بیشتر کاستیک و سولفات سدیم، حاکی از این مطلب است که بهترین دما و فشار عملیاتی ºC 150 و 5 بار میباشد.

فرایند شیرین سازی گاز مایع در فازهای 9 و 10 شرکت مجتمع گاز پارس جنوبی بر اساس فرایند سولفورکس انجام می گیرد که در آن از محلول سود جهت جداسازی مرکاپتانها و سایر ترکیبات سولفوردار استفاده میشود.محلول سود پس از جذب ترکیبات گوگردی در واحد احیای کاستیک تحت یک واکنش اکسیداسیون کاتالیستی احیا و مجددا در سیکل تصفیه گاز مایع استفاده می شود.با توجه به کارکرد بهینه واحد در بازه های زمانی کوتاه مدت ناگزیر از تخلیه حجم زیادی از Spent Caustic که حاوی محلول کاستیک 12-15% ، نمکهای کربنات سدیم و سولفات سدیم،مقادیری از دی سولفاید و مرکاپتان ها هستند، می باشیم.این مقاله در صدد است تا با بهره گیری از یک روش آزمایشگاهی در واحد احیای کاستیک و ایجاد یک تغییر فرایندی در واحد مرکاپتان زدایی پروپان به میزان بسیار زیادی از حجم کاستیک دورریز کاسته و به تبع آن علیرغم صرفه جویی اقتصادی، با کاهش پساب واحدهای مذکور تاثیر بسزایی در کاهش آلودگی های زیست محیطی داشته باشد.


Thermal wet oxidation processes
Thermal wet oxidation processes use high-temperature and high-pressure air or oxygen as oxidant. Wet air oxidation (WAO) refers to a process of oxidizing wastewaters, the water-containing liquid wastes under pressure with air or oxygen, and at high temperature (>120°C). It is a good option for treating the high-organic content PWWs which can originate from fine chemical and pharmaceutical industries. The chemistry of wet air oxidation involves chain reactions of radicals formed from organic and inorganic compounds present in the reaction mixture. In this hydrothermal process, the organic pollutants are converted into easily biodegradable substances or completely mineralized and the inorganic compounds are converted into their form with higher oxidation value, such as sulfides into sulfates.
A typical condition for wet air oxidation ranges from 180°C and pressure of 2 MPa to 315°C and 20 MPa pressure.
2.1.1. Industrial application of wet air oxidation
WAO technologies have been commercialized around 60 years ago. Applying different temperatures, one can mention three different oxidation categories, namely: low-temperature oxidation, medium temperature oxidation, and high-temperature oxidation.
Commercial application of low-temperature oxidation (100°C-200°C) involves low-pressure thermal conditioning (LPO) of activated sludge and the treatment of low-strength sulfidic spent caustic. The most prevalent WAO applications are for ethylene plants spent caustic and refinery spent caustic.
The ethylene plant spent caustic is traditionally oxidized at low temperatures in the range of 120°C-220°C; the main purpose is to destroy the odorous sulfide content of this effluent. The refinery spent caustic WAO application operates at 240°C-260°C that already fits the medium temperature category, as well as the WAO of some organic wastes. Other industrial wastes treated by low-temperature oxidation include cyanide and phosphorous wastes, as well as non-chlorinated pesticides. High-temperature oxidation 260°C-320°C is used for refinery spent caustic, sludge destruction, and most WAO treated industrial wastewaters. Most organic industrial wastes are oxidized in this temperature range, including pharmaceutical wastes as well as pesticides, solvents, and the complete destruction of liquid wastes of pulp and paper production and other organic sludges.
2.1.2. Zimpro® wet air oxidation and related processes
The history of WAO started almost about 60 years ago, when Zimmermann observed that he could dispose of pulp mill liquors using air at high pressure leading to the oxidation of organic compounds dissolved or suspended in water at relatively low temperatures in the presence of oxygen [1].
They took spent pulping liquor from a local paper mill to produce artificial vanilla flavoring (vanillin) by partial oxidation of ligno-sulfonic acids. They perfected the wet air oxidation process (or the “Zimmermann Process” as it was known), and expanded it to other applications, including wastewater treatment. The company, developed and installed these wet oxidation plants, had a diversified history as it was established by Sterling Drug Inc. as “Zimpro” at Rothschild in 1961, building the engineering and research center along the Wisconsin River. After a long expansion period, with new developments and acquisitions, Zimpro was purchased by USFilter in 1996. In April 1999, Paris-based Vivendi announced the acquisition of USFilter. Siemens bought USFilter in May 2004. Presently, it is owned by Siemens Co. under the name “Siemens Water Technologies.”
Since the beginning, this process (Zimpro) had been mainly used for sewage sludge treatment, but by the early 1970s, it was applied to regenerate spent powdered active carbon from wastewater treatment processes. During the 1980s, WAO began to be more useful as an industrial waste treatment technology. Zimpro Products installed the first wet oxidation unit in 1982 to treat the ethylene plant spent caustic. The next year, they installed and operated a wet oxidation system in California for the treatment and detoxification of hazardous wastes. In 1992, Zimpro installed a wet oxidation system at Sterling Organics in Dudley, Northumberland, UK, for pharmaceutical wastewater treatment. Currently, about 200 full-scale WAO plants are in operation for the treatment of a variety of effluent streams (municipal sludge, night soil, carbon regeneration, acrylonitrile process effluent, metallurgical coking, ethylene production spent caustic, paper filler, industrial activated sludge, pulping liquor, warfare chemicals, paper mill sludge, explosives, monosodium glutamate production, polysulfide rubber, textile sludge, chrome tannery waste, petroleum refining spent caustic, miscellaneous industrial sludges, nuclear reprocessing wastes) [2, 3]. In 2009, 2011, and 2012, Siemens contracted with Sinopec to build several WAO units in PR China for the disposal of spent caustic from ethylene plants and refineries.

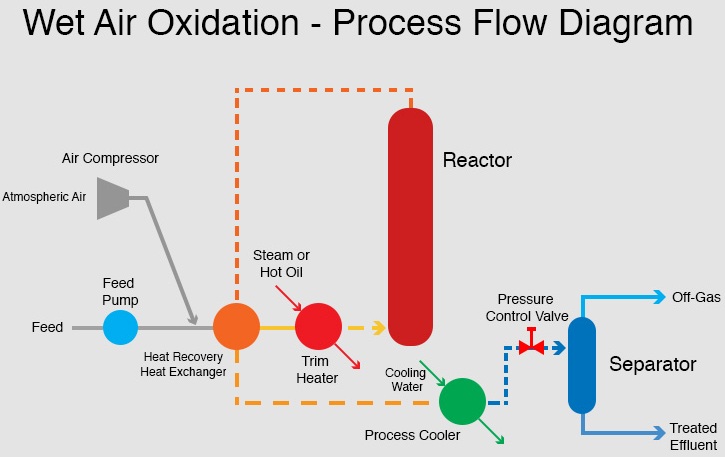
Figure 1.
Typical flow diagram of WAO [2]
The typical wet oxidation system (Figure 1) is a continuous process using a rotary compressor and pump to compress the air (or oxygen) and feed liquid stream to the required operating pressure. Heat exchangers serve to recover energy from the reactor effluent and use it to preheat the feed/air mixture entering the reactor. Auxiliary energy, usually steam, is necessary for startup and can provide trim heat if required. The residence time in the reactor vessel is several hours at a temperature that enables the oxidation reactions to proceed in some cases toward total mineralization. The reactor is a bubble column; it is coupled after the heat exchanger with a separator for the separation of the affluent and the off-gases. Since the oxidation reactions are exothermic, sufficient energy may be released in the reactor to allow the wet oxidation system to operate without any additional heat input at or above COD > ~10000 mg/L.
The typical reactions during WAO:
The oxidation reactions occur at temperatures of 150°C to 320°C and at pressures from 10 bar to 220 bar. The required operating temperature is determined by the treatment objectives. Higher temperatures require higher pressure to maintain a liquid phase in the system. Typical industrial applications for the WAO process have a feed flow rate of 1 m3/h to 50 m3/h per unit, with a chemical oxygen demand (COD) from 10,000 mg/L to 150,000 mg/L (higher COD with dilution).
During the decades of process development, huge amounts of data were collected and published about the oxidation properties of individual compounds, different process wastewaters, and sludges. In WAO of ethylene spent caustic, the conversion of sulfide is nearly 100% at 200°C for 60 minutes. The high conversion of the polluting compounds in refinery spent caustic needs higher temperature of oxidation (260°C), residuals from pesticide and herbicide production need even higher (280°C). This is associated obviously with higher total pressure because of the increased vapor pressure of water and other volatiles.
A good instance is, for illustrating the technical details and problems of WAO, the oxidation of ethylene plant spent caustic, which is one of the best-elaborated technology among WAO processes. Such spent caustic liquor contains as major components the compounds listed in Table 1. The sulfur-containing compounds are oxidized to sulfate, being present in the basic solution as sodium sulfate, the organic components are oxidized primarily to carboxylic acids, such as acetic, oxalic, formic, and propionic acid. At 200°C and 28 bar pressure, only the partial oxidation of organic compounds occurs, as the forming carboxylic acids are well biodegradable [4].
| Compound | Concentration range |
| NaHS | 0.5-6 % |
| Na2CO3 | 1-5 % |
| NaOH | 1-4 % |
| NaSR | 0-0.2 % |
| Soluble oil | 50-150 ppm |
| TOC | 50-1500 ppm |
| Benzene | 20-100 ppm |
Table 1.
Composition of ethylene plant spent caustic
WAO reliability can be hampered by off-spec feed, which is affected by the upstream processing and handling of the spent caustic. The feed of the WAO plant for treating ethylene plant spent caustic contains some reactive organic compounds (called “red oil,” main components are primarily aldehydes that form high molecular weight materials through the so-called aldol condensation reaction) that cause fouling not only in the ethylene compressor and the separation equipment but in the heat exchanger in front of the oxidation reactor serving for the preheating of the caustic feed and cooling of the effluent. The fouling is even worse in the presence of iron that usually forms an insoluble scale, which has to be removed by chemical and/or mechanical treatment. Chloride is dangerous because of corrosion, in spite of the use of special alloys in the WAO equipment.
A special process is the so-called VerTech oxidation of sludges. This applies an underground installation, consisting of concentric tubes as heat exchangers going down to 1200 m depth to an oxidation vessel. Due to the depth of the vessel, the bottom pressure in the reactor is above 100 bar at 275°C without the need for high-pressure pumps at the surface. The building of the installation required drilling and casing technology developed by the gas and oil production industry. The operation of the system required a frequent descaling operation with nitric acid in order to preserve the efficiency of heat exchange in the concentric tubes. The output of this plant was 80 tons of sludge per day [5].
2.1.3. Kinetic mechanism of WAO
WAO of organic pollutants is generally described by a free-radical chain reaction mechanism in which the induction period to generate a minimum radical concentration is of great significance [6-9]. In the most detailed studied reaction in WAO, in the oxidation of phenol water solution during the induction period, practically no change was observed in phenol concentration. Once the critical concentration of free radical is reached, the fast reaction takes place (propagation step) when almost all phenol is oxidized. It has been found that the induction period length depends on oxygen concentration, temperature, type of organic compound, and if applied on the catalyst concentration [2, 10-13]. The pH also has an influence on the induction period that actually is shorter for pH values of about 4, and is increasing with the increase in pH [14].
In wet oxidation, the reaction chains are thermally initiated [15]:
In the mechanism, R is an organic molecule present in the reaction mixture. In addition to the organic radicals several inorganic radicals participate in the degradation such as H, HO, and HO2/O2–. Among these radicals HO is the most reactive, it reacts with aromatic molecules (Ph=phenol) in radical addition reaction with practically diffusion-limited rate coefficient at all temperatures up to the supercritical value [16].
The hydroxycyclohexadienyl radical formed in Reaction (7) from phenol is also highly sensitive to oxygen.
In all real PWW and in the reaction mixtures of wet oxidation in stainless steel autoclaves, Fe ions are present with measurable concentration [17-19]. These ions and other transition metal ions present to accelerate the decomposition of peroxide to HO radicals in Fenton-type processes. The steady-state (propagation step) is then followed by the third step (termination step) characterized by a slow oxidation rate.
The first step is the chain initiation, in which free radicals (R, OH, HO2) are produced by dissociation (1) and the bimolecular reaction of dissolved oxygen with the organic compound (2), which is found to be very slow at low temperatures. When the free radical R is formed, it can readily react with molecular oxygen to give peroxoradical (ROO) (3). The other reaction is the formation of hydrogen peroxide, which decomposes with metal catalysis to OH radicals (6). Finally the peroxo radical gives with the parent compound a free radical and hydroperoxide (4). The OH radicals oxidize the parent compound into a free radical again (5).
In the mechanism, the organic parent compound (RH) can react thus with molecular oxygen, the organic peroxyl (ROO ) (5), hydroxyl (OH ) (6) and hydroperoxyl (HO2 ) (3) radicals [20, 21].
Intermediates formation is of great importance in WAO and has been reviewed by Devlin and Harris for the oxidation of aqueous phenol with dissolved oxygen [22]. The conclusion was that, at elevated temperatures, oxygen is capable of three different oxidation reactions with the organic: (i) introducing an oxygen atom into an aromatic ring to form a dihydric phenol or quinone; (ii) attacking carbon to carbon double bonds to form carbonyl compounds, and (iii) oxidizing alcohols and carbonyl groups to form carboxylic acids. The ring compound intermediates (dihydric phenols and quinones) were formed under conditions near the stoichiometric ratio of phenol and oxygen, increasing in quantity when oxygen was in deficiency. The unsaturated acids, namely maleic and acrylic and saturated ones, namely formic, acetic and oxalic appear independently of phenol to oxygen ratio used. Malonic, propionic, and succinic acids were identified only in case of deficit of oxygen. Malonic acid undergoes decarboxylation to produce acetic acid and carbon dioxide.
Another interesting research was done about the kinetics of oxidation of phenol, and nine substituted phenols were investigated [10]. The process was studied in a 1 L stainless steel autoclave at temperatures in the range of 150°C-180°C and the initial phenol concentration was 200 mg/L. The oxidation reaction found to be the first order for oxygen and also first order with respect to phenolic substrates in both cases. The overall oxidation reaction rate was found to be kinetically controlled when the temperature was less than 195°C and the phenol concentration was less than 200 mg/L. At higher temperatures (>240°C) and higher phenol concentration (>20000 mg/L) the overall oxidation reaction rate became mass transfer controlled.

Figure 2.
Proposed reaction pathway for phenol oxidation by molecular oxygen.
2.2. Catalytic Wet Air Oxidation (CWAO)
In the 80s significant need was revealed toward the treatment of highly concentrated wastewaters of chemical and pharmaceutical production, as well as residual sludge [22, 23]. Aside from WAO, CW(A)O has been applied to many different model effluents, but relatively few works have been devoted to real and complex industrial wastes [2, 24-28].
In order to carry out wet oxidation under milder conditions (at lower temperature and pressure) an alternative way would be catalytic wet (air) oxidation (CW[A]O). Soluble transition metal salts (such as copper and iron salts) have been found to give significant enhancement of the reaction rate, but post-treatment is needed to separate and recycle them. Heterogeneous catalysts have the advantage that they can be used without the problem of separation and for continuous operation. Mixtures of metal oxides of Cu, Zn, Co, Mn, and Bi are reported to exhibit good activity, but leaching of these catalysts was detected [25, 29-32]
The reaction mechanism of CWAO is thought to be similar to the mechanism of WAO, and the function of the catalyst is essential promoting the formation of free radicals.
A kinetic study was carried out on the phenol oxidation by CWAO using aqueous copper nitrate as a homogeneous catalyst. A kinetic model has been established based on the free radical mechanism where the electron transfer from copper to phenol was assumed to initiate the formation of free radicals and this led to propose that the formation of free radicals (PhO⋅ and PhOOO⋅) is primarily due to the electron transfer from metal to phenol. In this model, the reaction orders were found to be approximately 1.0, 0.5, and 0.5 with respect to phenol, oxygen, and copper concentrations, respectively. In order to verify the proposed kinetics, a series of CWAO experimental tests were done at 313-333 K, oxygen partial pressures of 0.6-1.9 MPa, and copper concentrations 0-13 mg L−1. The experimental data fitted well with the model [33].
With supported copper oxide catalyst in the temperature range of 120°C-160°C, with 0.6 MPa and 1.2 MPa oxygen pressures, it was found that the reaction was first order with respect to phenol concentration and half order with respect to oxygen partial pressure [34].
The composition of the most active heterogeneous catalysts in wet oxidation, namely that they are multicomponent, alludes on the validity of the Mars-van Krevelen mechanism well known in catalytic oxidation chemistry [35, 36].
2.2.1. Industrial homogenous CWAO processes
In these processes, homogeneous transition metal catalysts are used that need, however, to be separated and then recycled to the reactor or discarded.
The first process to be mentioned was developed by Ciba-Geigy, which uses a copper salt as a catalyst. From the oxidized material the catalyst has to be separated as copper sulfide and recycled into the reactor, which is titanium lined. The unit works at 300°C and pressure above 100 bar. Three units that are installed in Germany and Switzerland have achieved high oxidation efficiencies (95%-99%) on chemical and pharmaceutical wastes at elevated temperatures.
The other one is the LOPROX process, a relatively low-temperature and low-pressure wet oxidation that was developed by Bayer AG for the treatment of organic substances, especially aromatic compounds, which degrade too slow in normal biological plants or adversely affect the degradation of other substances. The disposal of aromatics is important for two reasons, they are forming a significant portion of PWWs from the chemical industry. They are present in the clarified activated sludge also, which contains humic acids, these have aromatic part with chlorine substituents. It takes place in the presence of oxygen in acidic range in a multi-stage bubble column reactor under relatively mild operating conditions (temperature below 200°C, pressure 0.5-2.0 MPa) the catalyst is the combination of Fe2+ ions and quinone-generating substances. The residence time is 1-3 hours. Above COD value of 6-8 g/L the process is autothermal, no heat energy input is needed. A critical issue is the choice of structural materials of the reactor and of other hot parts of the system. Enameled or PTFE lined steel can be used up to 160°C, titanium and titanium-palladium alloys are applied up to 200°C because of the acidic pH. Several LOPROX plants are in operation at Bayer AG. They dispose of PWWs from intermediate, dyestuff, pharmaceutical, paper, and pulp production, and clarified sludge.
Veolia developed the ATHOS® process for the treatment of clarified sludge at WWTPs. It works with Cu ion catalysis at 250°C and 50-60 bar pressure with pure oxygen. The reactor is perfectly mixed because it has a circulation loop, not the usual bubble column. The heat exchangers are working with extremely high-temperature water as heating source. Such plant is working at the Brussels WWTP, in a complex line for the abatement of clarified sludge [37].
2.2.2. Industrial heterogeneous CWAO processes
The development of stable and active heterogeneous catalysts for WO of PWWs is a difficult task, as the substrates to be oxidized are diverse, the wastes are multicomponent, and severe conditions are needed for the completion of the reactions. At high-temperature and high-oxygen partial pressure even at the basic pH of the reaction mixture the leaching of the active component(s) of the catalyst into the water solution frequently occurs. As mentioned in a review, there are two catalytic WAO technologies that have been developed in the late ‘80s in Japan. Both processes use heterogeneous catalysts, precious metals deposited on titania-zirconia carriers. They are able to oxidize two refractory compounds namely acetic acid and ammonia also [27].
The NS-LC process uses a Pt-Pd/TiO2-ZrO2 honeycomb catalyst. Typical operating conditions are 220°C and 4 MPa pressure with space velocity = 2 hour-1, which with these operating conditions the oxidation of compounds such as phenol, formaldehyde, acetic acid, glucose, etc., exceeds 99% conversion. In the absence of a catalyst, the removal efficiency would go down to 5%-50% [38]. The specialty of the reactor is the segmented gas-liquid flow, which means that the liquid plugs are sandwiched between two gas plugs, and the flow has a mass transfer increasing and solid deposition preventing effect.
The other process, which is called Osaka Gas, is based on a mixture of precious and base metals on titania or titania-zirconia carriers in a form of honeycomb or sphere. This process has been applied in several industrial and urban wastes. A typical pilot plant at British Gas’s London Research station works at 250°C and pressure of 9 MPa, with 200 L/h feed of waste [39].
Kurita Company developed a process to abate ammonia with the oxidation agent nitrite in the presence of a supported Pt catalyst. The reaction temperature (170°C) is lower than in the usual WO.
One of the recent developments is the CALIPHOX process made by the National Institute of Chemistry of Slovenia and an engineering firm for the treatment of industrial wastewaters with a metal oxide catalyst in the extruded form in a trickle-bed reactor. It is operating in relatively mild conditions (180°C, 4 MPa). The catalyst is based on the work of Pintar and Levec [40] who studied CuO- ZnO-Al2O3.
2.2.3. Types of CWAO catalysts
The objective of catalyst application, beside reaction rate enhancement, is to operate among milder conditions. The catalyst is usually a metal salt, a metal oxide, or the metal itself [Table 2.]. As we know, heterogeneous catalysts based on precious metals deposited on stable supports are less sensitive to leaching [25, 31, 41-44]. Different catalysts were applied and their effects were investigated on different catalytic wet oxidation processes in the past years. Pt and Ru on ceria and zirconia-ceria supports were tested in the oxidation of acetic acid that was accompanied by the loss of activity [32]. In the following paper, the same authors described the reason for the deactivation of the Pt catalyst, the accumulation of carbonate species on the surface [45]. Recently, the activity of Ru-oxide on different oxide supports in acetic acid oxidation was reported. The mixed Zr, Ce oxide supported catalyst proved to be the most active [46].
| Substrate | Reaction condition | Oxidant, catalyst | Reactor type | Removal | Reference |
| Phenol | 120-160°C 6-12bar(O2) |
Air, Cu | trickle bed reactor | <90%COD | Fortuny et al.1999 |
| Phenol, acetic acid | 170-200°C 20 bar (O2) |
O2, Ru, Pt, Rh | batch | <97%COD | Duprez et al. 1996 |
| Phenol chlorophenol nitrophenol |
150-210°C 30 bar (total) |
O2, CuO, Zn, Co oxides | trickle bed reactor | >95%TOC | Pintar&Levec1994 |
| Phenol | 90-150°C | CuO+Al2O3 O2, CuO+ZnO |
batch | 100% X | Akyurtlu et al.1998 |
| p-chlorophenol | 180°C 26bar (total) |
O2,Pt, Pd,Ru | slurry | <98% TOC | Qin et al., 2001 |
| Ammonia | 110°C-130°C | O2, Pt/SDB | trickle bed reactor | 100% X | Huang et al., 2001 |
| Ethylbenzene | 310-390°C 0-1 bar (O2) |
O2, AC | packed bed reactor | 50% | Pereira, et al, 2000 |
| Aniline | 160-230°C 20 bar O2 |
O2, Ru/CeO2 | batch | 100% X | Oliviero et al., 2003 |
| Paper industry wastewater |
140-190°C 5 bar (O2) |
O2, Cu/Mn, Cu/Pd, Mn/Pd |
batch | >84% TOC | Akolekar et al., 2002 |
| Carboxylic acid | 180°C 1-11bar(total) |
Air, Pt/Al2O3 | batch | 100% X | Lee & Kim, 2000 |
| Kraft bleach plant effluent | 190°C 8 bar (O2) |
Air, Ru/TiO2 | trickle bed reactor | < 90%TOC | Pintar et al., 2001 |
Table 2.
CWAO of organic pollutants and industrial effluents. [40-41, 47-56]
2.2.3.1. Supported noble metal catalyst
Various noble metals (Ru, Pt, Rh, Ir, and Pd) and some metal oxides (Cu, Mn, Co, Cr, V, Ti, Bi, and Zn) have traditionally been used as heterogeneous catalysts in CWAO. Several studies have ranked catalysts according to their activity. Imamura and his colleagues ranked noble metal and metal oxide catalysts according to their total organic carbon conversion achieved in 1 h, during the oxidation of polyethylene glycol at 200°C and pH of 5.4 [57]. They found the following order: Ru = Rh = Pt > Ir > Pd > MnO.
2.2.3.2. Supported metal oxides
Metal oxides can be classified according to their physical-chemical properties. One of these properties is the stability of metal oxide. Metals with unstable high oxidation state oxides, such as Pt, Pd, Ru, Au, and Ag do not perform stable bulk oxides at moderate temperatures. Most of the commonly used metal oxide catalysts (Ti, V, Cr, Mn, Zn, and Al) have stable high oxidation state oxides. Fe, Co, Ni, and Pb belong to the group with intermediate stability of high oxidation state oxides [58].
Mixtures of metal oxides frequently exhibit greater activity than the single oxide. Cobalt, copper, or nickel oxide in combination with the following oxides of iron (III), platinum, palladium, or ruthenium, are reported as effective oxidation catalysts above 100°C [59]. In addition, combining two or more metal catalysts may improve non-selective catalytic activity.
Metal oxides are usually applied in the form of powders and fine particles, and with this form of catalyst structure we can achieve maximum specific surface area, but the dispersion of the particles can create an unsteady state. To keep the stable state of the catalyst, at the same time not losing the active phase, some porous supports can be used. Commonly, alumina and zeolites are used as support, but the surface area of aluminum oxide is limited and the pore size of zeolites cannot be permeable for large size organic molecules.
2.2.3.3. Activated carbon catalysts for CWAO
Another promising catalyst could be activated carbon (AC) that shows good properties as an adsorbent for both organic materials and oxygen because of its porous structure and high surface area [60, 61]. Activated carbon is stable in highly acidic and basic media and it is also easy to prepare, which is why it is used as a catalyst for different reactions [62], and also as a support for other oxidation catalysts [63, 64].
Activated carbon can also catalyze the polymerization reactions in the presence of oxygen via an oxidative coupling. Phenol oxidation over activated carbon in the trickle bed reactor has been investigated [65, 66]. The activated carbon was found less active than metal oxide catalysts but more stable and more environmentally accepted, and of course cost-effective [65, 66].
Phenol conversion was compared using the copper catalyst and activated carbon [67]. In the long run, the copper catalyst was found to lose its activity due to the leaching of the copper phase. On the other hand, activated carbon also exhibited a continuous drop in phenol conversion, starting from nearly complete and finally reaching about 48%. However, the loss of activated carbon efficiency could be ascribed to its consumption during experiments; thus, the absolute activity of activated carbon remained stable during the long term.
One of the recent studies was about the CWAO of paracetamol on activated carbon [68]. The CWAO of paracetamol was investigated both as a water treatment technique and as a regenerative treatment of the carbon after adsorption in a sequential fixed bed process. They used three ACs as catalysts: a microporous basic AC and meso- and micro-porous acidic ACs. During the first CWAO experiment, they noticed that the adsorption capacity and catalytic performance of fresh basic activated carbons (S23 and C1) were higher than those of the fresh microporous acidic one (L27) despite its higher surface area. It seems that this situation changed after reuse, as finally, L27 gave the best results after five CWAO cycles. Respirometric tests were also done with activated sludge and it was mentioned that in the studied conditions the use of CWAO enhanced the aerobic biodegradability of the effluent. The group also checked the different aging by measuring the physicochemical properties of activated carbons.
2.2.4. Application of CWAO in the fine chemical industry
CWAO has been applied to many different model effluents, but relatively few studies have been devoted to real and complex industrial wastes [23, 27-28, 69-73]. Even in literature, there is very limited number of works that dealt with real complex wastewaters. Our focus here is the pharmaceutical industry that produces mixtures of liquid wastes containing water and organic solvents, aside from higher molecular weight organic and inorganic compounds with different concentrations and different pH. Treating these wastewaters needs special conditions.
In a publication, the catalytic wet oxidation of wastewater originating from apramycin production was investigated with supported Ru oxide catalysts [72]. Ru catalyzed oxidation of wastewaters originating from meat processing and vegetable processing industries were also carried out [73]. Three rather detailed reviews were published concerning wet oxidation and catalytic wet oxidation [60, 74, 75]. They also mentioned the oxidation of miscellaneous organic compounds, but published no data specifically about the oxidation of pharmaceutical PWWs.
In another research, the effect of CuO/Al2O3 was investigated—which was prepared by consecutive impregnation—on three different azo dyes (Methyl Orange, Direct Brown, and Direct Green), which were treated by CWAO. The relationships of decolorization extent, COD, and total organic carbon (TOC) removal in the dye solution were also investigated. The 99% of color and 70% of TOC removal in 2 h indicated that the CuO/Al2O3 catalyst had excellent catalytic activity in treating azo dyes [76]. In Table 3, the most characteristic results of CWAO are collected; the substrates tested are in most cases phenol and acetic acid. The latter is resistant in WO, but easily biodegradable in the biological denitrification.
| Catalyst | Application | ||
| Active Phase | Carrier | Substrate | Reference |
| Cu | Alumina | Phenol Phenol p-cresol |
Sandra et. al. 1974 Kim et al. 1991 Mishra et. al. 1993 |
| Cu | Alumina Silica |
chlorophenols | Sanger et. al. 1992 |
| Cu-Zn-Al oxide | Alumina | phenol compounds | Pintar et al. 1992 |
| Cu-Mg-La | Zn aluminate | acetic acid | Box et.al. 1974, Levec et. al. 1976 |
| Mn | Alumina Sr115 |
phenol chlorophenols |
Sandra et. al. 1974 Sanger et. al. 1992 |
| Mn-Ce | None | polyethylene-glycol | Imamura et al.1986 |
| Mn-Zn-Cr | None | industrial wastes | Moses et al. 1954 |
| Cu-Co-Ti-Al | Cement | phenol | Schmidt et. al. 1990 |
| Co | None | alcohols, amines, etc. | Ito et. al. 1989 |
| Co-Bi | None | acetic acid | Imamura et. al.1982 |
| Co-Ce | None | ammonia | Imamura et. al. 1985 |
| Fe | Silica | chlorophenols | Sanger et. al. 1992 |
| Ru | Cerium oxide | alcohols, phenols, etc | Imamura et. al. 1988 |
| Ru-Rh | Alumina | wet oxidized sludge | Takahasi et. al. 1991 |
| Pt-Pd | Titania-zirconia | industrial wastes | Ishii et. al. 1991 |
| Ru | Titania-zirconia | industrial wastes sludge | Harada et. al. 1993 |
| Pt | Alumina | phenol | Hamoudi et. al. 1998 |
| Mn | Cerium oxide | phenol | Hamoudi et. al. 1998 |
| Ru | Titania | phenol | Vaidya et. al. 2002 |
| Pt, Ru | Carbon black composite Silica-titania | phenol | Cybulski et. al. 2004 |
| Ru | Pelletized cerium oxide Zirconia | phenol | Wang et al. 2008 |
| Ru oxide | Titania Zirconia Titania-cerium oxide Zirconia-cerium oxide |
acetic acid | Wang et. al. 2008 |
| Pt, Ru | Cerium oxide, Zr-(Ce-Pr)-O2 | phenol | Keav et. al. 2010 |
| Ru, Ru-Ce | Alumina | isopropyl alcohol phenol acetic acid DMF |
Yu et. al. 2011 |
| Graphene oxide (GO) | None | phenol | Yang et. al. 2014 |
| Ru, Pt | Zirconia Cerium oxide Titania |
acetic acid | Lafaye et. al. 2015 |
| Ru, Pt | Titania Cerium oxide Titania-cerium oxide |
phenol | Espinosa de los Monteros et. al. 2015 |
Table 3.
Summary of reported heterogeneous catalytic WO research [38, 57, 77-100]
2.3. Intensification of WAO with combined technologies; effect of high energy radiation on wet oxidation at elevated temperature (the combination of the two methods)
As it is indicated in the previous section, one of the new areas in treating liquid wastes of high-organic content generated by the fine chemical mainly the pharmaceutical industry is the combination of AOPs with WAO. This area of work is rather new and there are very few articles dealing with this area of research.
We already know that the organic compounds undergo chain type oxidation in a reactor at high temperature and high pressure, of course sometimes in the presence of a catalyst. The high temperature is needed for the initiation of oxidation processes, but at the same time, this high temperature and pressure could cause serious corrosion even at alkaline pH, so it could be a good idea to decrease the temperature yet induce the chain initiation of oxidation by radiation. For this reason, WO and irradiation could be combined.
For radiation processing of polluted water, high-energy electron beam, γ -rays, or x-rays can be principally used. For producing the electron beam, electron accelerator devices are far the best radiation sources with respect to their output power and practical applicability.
2.3.1. Water radiolysis
In the interaction between ionizing radiation (high-energy electron beam, gamma rays) and water, electronically excited and ionized molecules are formed and the product of it will be primary species such as ⋅OH, e–aq, H⋅, and molecular products such as H2, H2O2. In the presence of oxygen in the water, the reducing species H-atoms and the solvated electrons (e–eq) are converted into oxidizing species, perhydroxy radicals (HO2⋅), and perhydroxyde radical anions (HO2⋅). The last one together with the OH⋅ radicals are responsible for the degradation of water pollutants.

Figure 3.
Radiolysis of water.
The radiation-induced degradation of neutral phenol solution was studied in the past using end-product techniques [101-105]. There are other few works on mechanistic studies on phenol and other aromatic molecules that were carried out by combining end products and transient detections and it was suggested that the transformations were initiated by hydroxyl radical attachment to the ring and reaction of O2 with the radicals produced [106-108].
Pulse radiolysis of 2,6-dichloroaniline in dilute aqueous solution was investigated. It is known that mono- and dichloroanilines are considered to be highly hazardous pollutants in wastewater. These compounds are important chemical intermediates of dye and plant protection agent production. In this investigation, the hydroxyl radical formed in water radiolysis was reacted with 2,6-dichloroanliline forming hydroxy-cyclohexadienyl derivative. The irradiation was carried out at room temperature by a 60Co γ-source, built into a panorama irradiator, with 1.5 kGy h-1 dose rate. The hydroxy-cyclohexadienyl radical in the absence of dissolved O2 partly transformed to aniline radical when oxygen was present in the reaction mixture the radical transformed to peroxy radical. According to chemical oxygen demand measurements, the reaction of one OH⋅ radical-induced the incorporation of 0.6 O2 into the products [109].
The irradiation-induced decolorization and degradation of aqueous solutions of azo dyes and some intermediates (anilines, phenols, triazines) were successful with electron beam irradiation. The experimental methods were the pulse radiolysis and end-product analysis with HPLC-MS. Demonstrating the practical applicability of this method, a continuous irradiation device has been built. The feed was the dye-containing water of red color; the effluent was the colorless liquid during contact time of less than 0.1 seconds with the high energy electron beam (4 MeV) generated by a linear electron accelerator [110].
The other topic is the investigation of the degradation of pharmaceuticals that can be detected in natural waters as emergent pollutants. The radiation-induced degradation of ketoprofen in dilute aqueous solution has been tested. The intermediates and final products of ketoprofen degradation were determined in 0.4 mmol/dm3 solution by pulse radiolysis and gamma radiolysis. UV-Vis spectrophotometry and HPLC separation served for identifying the product compounds [111].
The successful degradation of organic molecules being in small concentration in waters, with high-energy irradiation generating radicals already at room temperature prompted us to combine this method with WO. Recently, this hybrid method was used and compared with the classical WO method. The noticeable conversion was observed in phenol oxidation by irradiation already at room temperature in the presence of a high concentration of dissolved oxygen [112].
In one of the recent studies, WAO of highly concentrated emulsified wastewater was conducted. These kinds of wastewater usually contain all kinds of organic matters such as surfactants, additives, and mineral oils. They are typical, highly concentrated hardly biodegradable organic wastewaters. This oxidation took place in a 2 L high-pressure autoclave in batch mode. The initial COD concentration of the wastewater was 48000 mg/L. After 2 h of oxidation at 220°C with supply of oxygen 1.25 times more than its theoretical value, the COD was reduced by 86.4%. The temperature seemed to be a key influential factor, especially between 180°C and 220°C the COD and TOC removal was evidently increased. They also recognized that with increasing the initial partial pressure of oxygen (pO2), the reaction rate significantly increased [113].